International health experts have recently identified the drug tenofovir, lamivudine and dolutegravir (TLD) as the preferred first-line ARV for HIV treatment. In Haiti, the USAID Global Health Supply Chain Program-Procurement and Supply Management (GHSC-PSM) project is helping the Ministry of Health (MOH) transition health facilities to this preferred drug, and away from using legacy ARV regimens. GHSC-PSM has helped Haiti plan, prepare and execute its move to TLD since November 2018.
Making TLD and other commodities available to PLHIV in health facilities across the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) target countries remains a primary goal of GHSC-PSM—the project supports countries in many ways so they can begin the new and optimized treatment for patients as soon as possible.
In Haiti, one of the first elements of the TLD transition process was a month-long training for health care providers who treat PLHIV. The training covered the MOH’s new protocols and guidance that clinicians and providers are required to follow during the TLD transition. It was conducted for health facilities in all ten of the country’s administrative regions. The MOH also worked with GHSC-PSM in Haiti to conduct TLD supply planning, procurement and delivery.

The global effort to optimize antiretroviral (ARV) treatment is an ongoing commitment to people living with HIV (PLHIV) to improve the efficacy, reduce the costs, and limit the side-effects of their treatment.
What's a Legacy ARV?
Legacy first-line ARVs are those which may have been recommended by health officials as the primary option to treat PLHIV prior to the endorsement of TLD by WHO and other global health bodies. They include tenofovir, lamivudine and efavirenz (TLE).
By April 2019, 123 of 126 health facilities supported by PEPFAR were fully stocked with TLD—this makes up more than 97 percent of all PEPFAR-funded health facilities in the country.
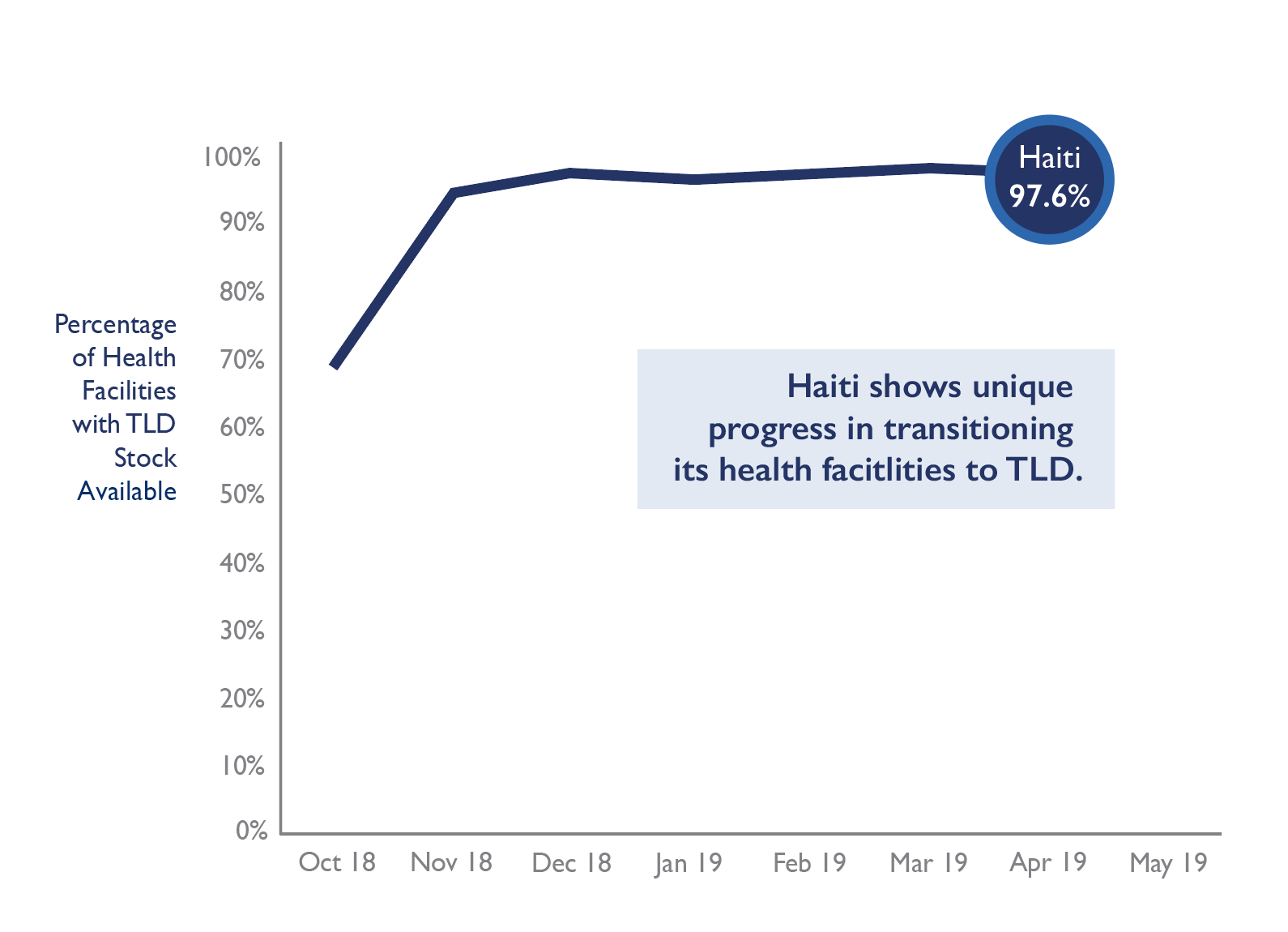
The deliveries facilitated by GHSC-PSM are expected to cover treatment for 43,232 patients.
To achieve such high availability of TLD for patients and health workers across Haiti’s health facilities, GHSC-PSM in Haiti worked with the MOH to:
- Implement an electronic system that manages and performs calculations with ARV consumption data, capturing and analyzing information on all patients eligible to receive TLD – the system works in tandem with health facility reporting processes and allows GHSC-PSM to automatically calculate the amount of TLD to allot per patient, thus revealing how much stock to provide per health facility;
- Organize a special distribution of TLD to health facilities, ensuring the product was available at all targeted sites prior to the transition start date;
- Increase the amount of TLD stock given to sites to facilitate Multi-Month Dispensing to patients, allowing them to go longer between clinic visits – the amount distributed per site was increased from 4.5 months of stock available to 7.5 months.
GHSC-PSM in Haiti continues to supply facilities with TLD according to the country’s transition plan. The project is also working with the MOH to address challenges in transitioning individual patients from one regimen to another. This includes mobilizing all the necessary stakeholders to move this transition forward. The MOH and GHSC-PSM have taken a grassroots approach by visiting individual communities, meeting with health facility staff, and engaging the local medical community. The goal is to spread accurate information about the benefits of changing patient regimens and emphasize how TLD can save the lives of patients.
Through uninterrupted supply of TLD; well thought-out solutions to challenges like access for patients and infrastructure challenges; and continued work with key stakeholders, Haiti will sustain its success in improving outcomes for people living with HIV.