MTaPS helped facilitate the prioritization and development of the EVML and guidelines on infection prevention and use of antimicrobials in Uganda, convening and coordinating the process across all stakeholders, including the Ministry of Agriculture, Animal Industry and Fisheries (MAAIF); the National Drug Authority; Makerere University; and the National AMR Sub-Committee. These developments are key to laying a foundation for antimicrobial stewardship in the agricultural sector. The newly developed and validated guidelines on infection control and use of antimicrobials in the animal health sector cover all leading animal production systems in Uganda (fish, poultry, sheep and goats, cattle, and pigs) and are made available on MAAIF’s website.
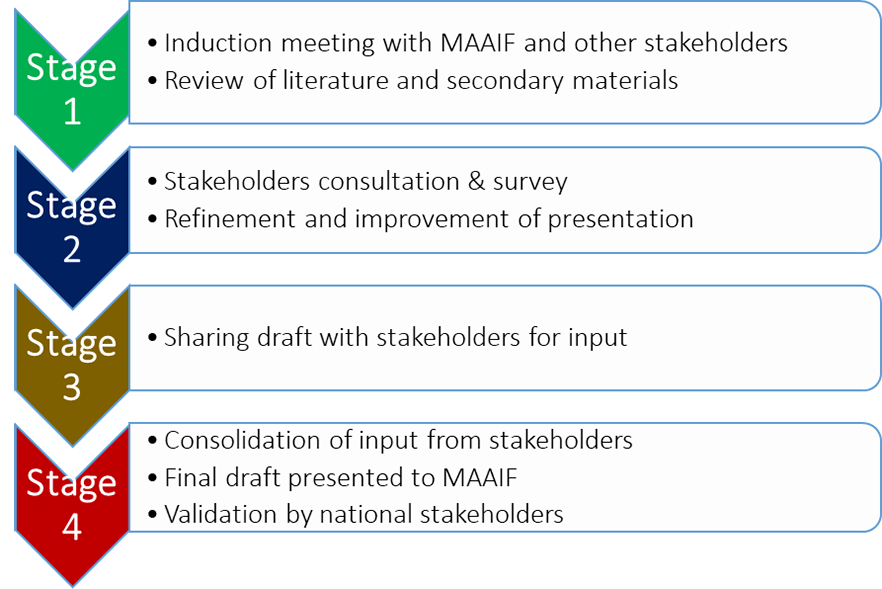
The development of the EVML and guidelines on the use of antimicrobials was a ground-breaking venture for the agriculture sector. The success was an outcome of a year-long participatory process from consultations to consolidation of input and validation by all national stakeholders (figure 1). The aim of the EVML is to guide policy and regulation on importation of essential medicines into the country, and it took into consideration current national and global recommendations by the tripartite collaboration of OIE, WHO, and the UN’s Food and Agriculture Organization on the use of antimicrobials in the agricultural sector. The guidelines for infection control and antimicrobial use will guide farmers on biosecurity on the farm, appropriate antimicrobial use, and safety of animals, thus increasing their agricultural productivity.
‘’Colleagues from the Ministry and other sectors join me in thanking USAID MTaPS for taking leadership in the development of this key policy document. For long we have struggled with the use of antibiotics and availability of medicines and control of their access in the agricultural sector. We now have a basis for implementing the changes.” – Dr. Anna Rose Ademun, Chief Veterinary Officer and Commissioner, Animal Health, MAAIF
The EVML and the infection prevention guidelines will regulate access to essential antibiotics, ensuring animal health and welfare while reducing their misuse. They also lay the foundation for cross-sectoral collaboration to implement Uganda’s AMR National Action Plan, thus advancing the Global Health Security Agenda.
Figure 1: Process of development of the EVML and guidelines on infection control and use of antimicrobials in the animal sector
Check out the newly developed Essential Veterinary Medicines List and guidelines informing and controlling the use of antimicrobials in the animal sector in Uganda on the Ministry’s website.